The VersaCross Connect™ Access Solution for FARADRIVE™ connects VersaCross™ technology with the FARADRIVE Steerable Sheath for an integrated zero exchange left heart access workflow.
- Overview
- Clinical data
- Technical specifications
- Resources
Zero exchange FARADRIVE Steerable Sheath delivery
For use with the FARAPULSE™ PFA Platform
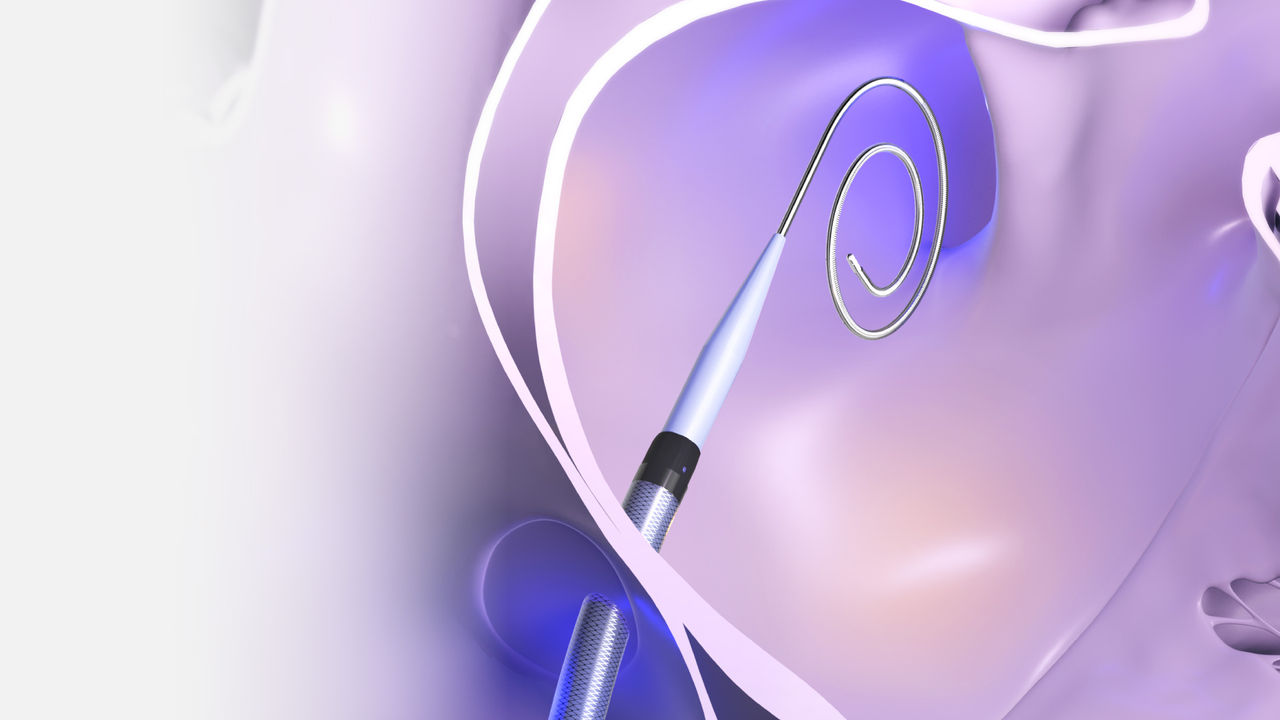
How it works
The VersaCross Connect Access Solution for FARADRIVE connects VersaCross technology with the FARADRIVE Steerable Sheath* for an integrated zero exchange left heart access workflow in your FARAPULSE Pulsed Field Ablation procedures.
Why choose the VersaCross Connect Access Solution for FARADRIVE
All-in-one transseptal access solution designed for use with the FARADRIVE Steerable Sheath*
Know where you are at all times with OMNIviz™ technology
Engineered to reliably locate the VersaCross Connect solution on fluoroscopy and ultrasound. Positional markers indicate position of radiofrequency (RF) tip within dilator. Connect to DuoMode™ Cable to track and mark RF tip on mapping system.
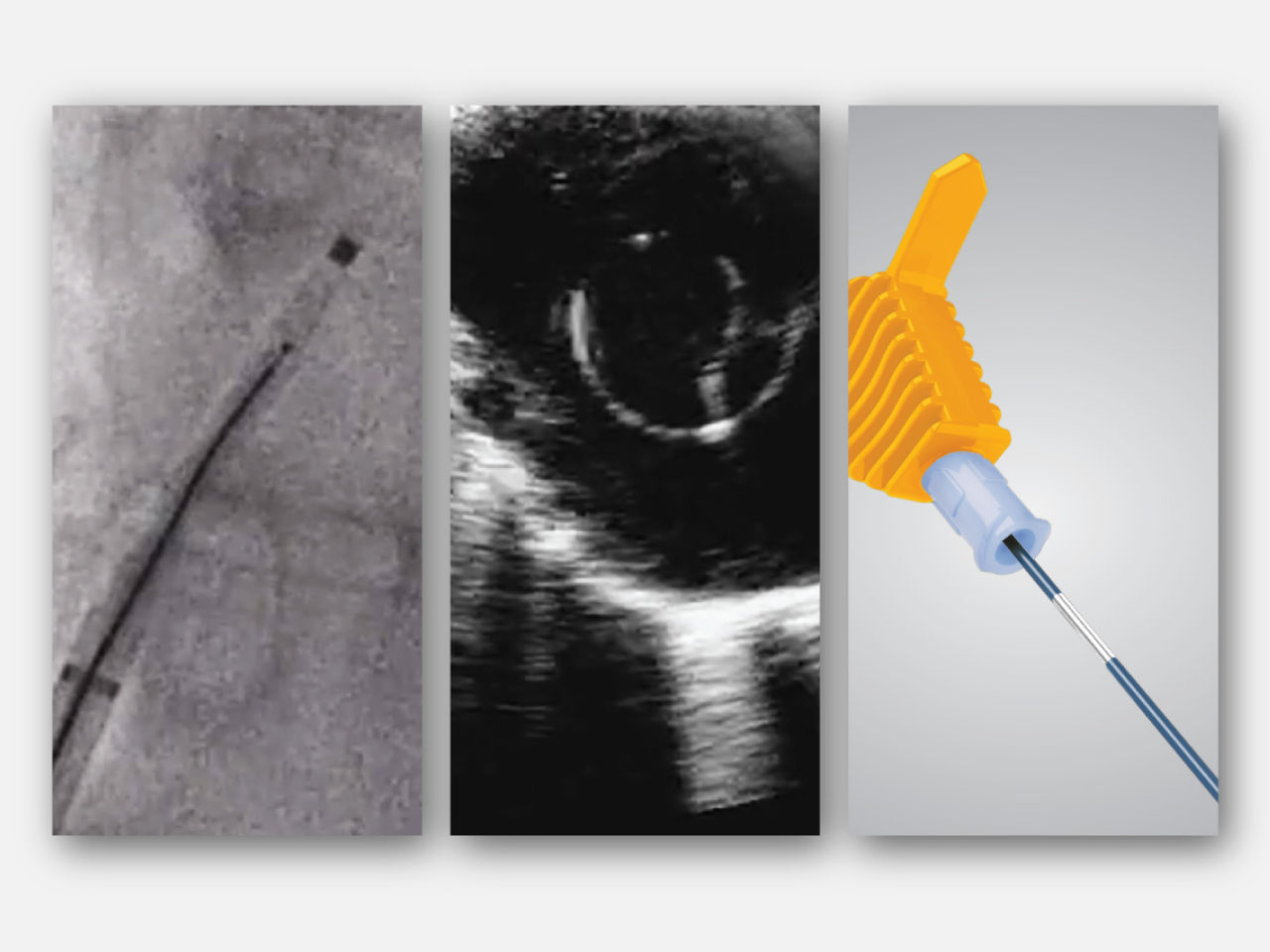
What’s included
The VersaCross Connect Access Solution for FARADRIVE includes:
- VersaCross RF Wire (j-tip or pigtail)
- VersaCross Connect Transseptal Dilator (13F) with TRUform technology
- Single-use RFP-100A™ Connector Cable
Required product

Designed for controlled tissue puncture using radiofrequency energy
Publication summaries
March 2025, Osorio et al., published in Journal of Cardiovascular Electrophysiology
VersaCross Connect Access Solution for FARADRIVE facilitated 100% transseptal crossing success in FARAPULSE Pulsed Field Ablation System procedures.
VersaCross RF Wire
| Feature | Specifications |
| Tip configurations | J-tip, pigtail |
| Curve shape | 9 mm (j-tip), 24 mm (pigtail) |
| Wire diameter | 0.035 in (0.89 mm) |
| Overall length | 180 cm |
VersaCross Connect Transseptal Dilator
| Feature | Specifications |
| Outer diameter | 13 F (4.4 mm) |
| Inner diameter | 0.036 in (0.9 mm) |
| Usable length | 93 cm |
| Curve | D1 |
Sheath compatibility
The VersaCross Connect Transseptal Dilator is for use with a 13F ID FARADRIVE Steerable Sheath which is 74 cm in length, specifically, model: M004PF21M402.
Brochure
References
*Optimized Biphasic Cohort
- Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021 May;7(5):614-27.
- Reddy VY, Neuzil P, Koruth JS, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019 Jul 23;74(3):315-26.
Indications, safety and warnings
FARAWAVETM Pulsed Field Ablation Catheter
FARASTARTM Pulsed Field Ablation Generator
FARADRIVETM Steerable Sheath
All trademarks are property of their respective owners.
CAUTION:
All content on our websites is provided for informational purposes only and is not intended or recommended as a substitute for professional medical advice. We may also include certain information, reference guides and databases intended for use by licensed medical professionals. These tools are not intended to give professional medical advice. Physicians and other health care providers should always exercise their own clinical judgment for any given situation. Boston Scientific does not promote or encourage the use of its devices outside of their approved labeling.
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.
You will be directed to websites that are located outside of Australia & New Zealand, which may include information on therapies or products not approved for use in Australia and/or New Zealand. In proceeding to this web page, you acknowledge that Boston Scientific Australia & New Zealand has not reviewed the content of the material contained through this link.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
Individual symptoms, situations, circumstances and results may vary. This website is meant for information purposes only, it is not intended to be used for medical diagnosis or treatment or as a substitute for professional medical advice. This site is intended for Australian and New Zealand residents only. Please review the Boston Scientific Privacy Policy for practices on the collection, storage, use and disclosure of your personal information.
Videos provided are meant for informational purposes only and may not be indicative of actual size or clinical outcome. The opinions, procedures and patient care policies expressed or depicted in this video are those of the physician and do not necessarily reflect the opinions, policies or recommendations of Boston Scientific Corporation or any of its employees. For further information and detailed instructions regarding the use of the Boston Scientific products featured in this video, please refer to the directions for use included within the Boston Scientific product package.
CAUTION: Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com.