ADVENT Pivotal Trial
ADVENT was a randomised clinical trial that directly compared FARAPULSE™ PFA to standard-of-care thermal ablation devices (force-sensing radiofrequency ablation —RFA—or cryoballoon ablation—CBA) for the treatment of paroxysmal atrial fibrillation (AF).*
*In Australia, the FARAWAVE PFA Catheter is indicated for isolation of pulmonary veins for the treatment of drug refractory paroxysmal atrial fibrillation.
It was a multi-centre, prospective, non-inferiority clinical trial with 1:1 randomisation of PFA to thermal ablation evaluating single-procedure, off-drug study endpoints, including:1,2
- Primary Safety
- Primary Effectiveness
- Procedural Characteristics
The most rigorous PFA clinical trial to date:
- Patients randomised to PFA or thermal ablation (RFA or CBA)
- Re-ablations not allowed in 90-day blanking period
- Freedom from Class I/III anti-arrhythmic drug (AAD) after the 90-day blanking period (amiodarone was not allowed at any time)
- Stringent monitoring with 72-hour Holters
- Largest PFA trial with 305 patients treated with PFA
Trial results were collected from 65 thermal operators in 30 centres with 607 randomised patients treated with PFA or thermal ablation and included in the primary results.
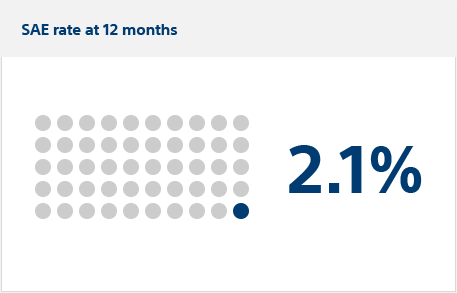
Primary safety endpoint:
Severe adverse events (SAEs)
Serious adverse events occurred in six FARAPULSE PFA patients (estimated incidence: 2.1%) vs. four thermal patients (1.5%), meeting the criterion for non-inferiority (posterior probability >0.999).
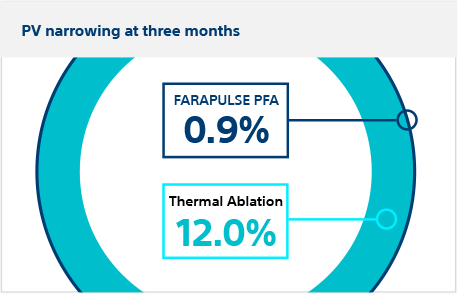
Secondary safety endpoint:
Pulmonary vein narrowing
Patients returned at three months for CT or MRI scan. There was significantly less pulmonary vein cross-sectional narrowing in FARAPULSE PFA patients (0.9%) vs thermal ablation patients (12.0%), meeting the criterion for superiority (posterior probability >0.999)
ADVENT is the first and only randomised clinical trial in which PFA has been shown to be non-inferior* to standard-of-care thermal ablation (RFA and CBA) for primary safety endpoints.
*(posterior probability >0.999)
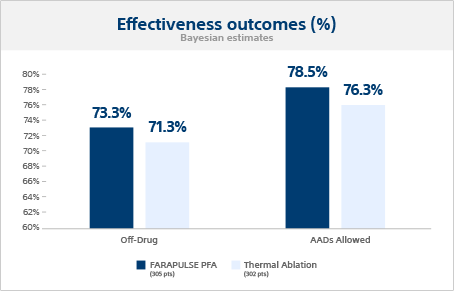
Effectiveness outcomes
In the ADVENT trial, the primary effectiveness endpoint required both acute procedural success (acute PVI) and chronic success, which included freedom from Class I/III AADs, repeat ablation, cardioversion, and documented AF, AFL, or AT through 12 months. The investigators were experienced thermal operators with limited FARAPULSE PFA device experience. The FARAPULSE 12-month, off-AAD effectiveness outcome was non-inferior (posterior probability >0.999) to thermal devices. The acute PV isolation rates were 99.6% for FARAPULSE and 99.8% for thermal ablation.
Primary effectiveness outcome
The Bayesian estimated single-procedure, off-drug treatment success probabilities were 73.3% for FARAPULSE and 71.3% for thermal ablation, meeting the criterion for non-inferiority.
Additional effectiveness outcome
When anti-arrhythmic drugs (AADs) were allowed post-blanking, the 12-month success probabilities were 78.5% for FARAPULSE and 76.3% for thermal ablation.
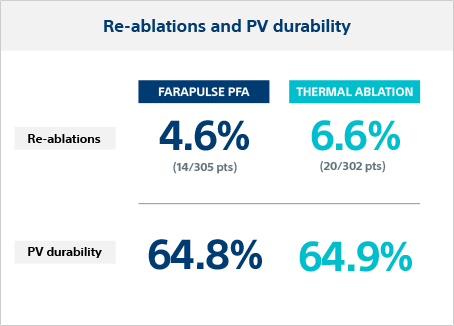
PV durability in re-ablation
Repeat ablation was performed in 4.6% of FARAPULSE patients and 6.6% in thermal ablation patients. The PV durability in re-ablated patients was 64.8% per vein (28.6% per patient) for FARAPULSE and 64.9% per vein (26.3% per patient) for thermal.
The ADVENT FARAPULSE 12-month effectiveness outcome was non-inferior* to thermal ablation devices, although operators had limited experience with the PFA system and were considered expert thermal operators.
*(posterior probability >0.999)Procedural characteristics
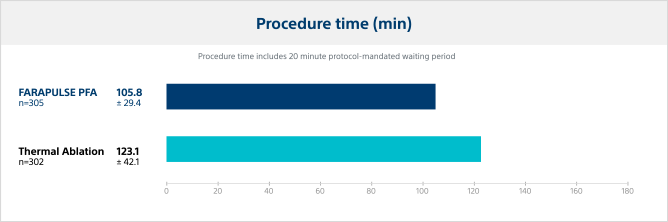
In addition to the primary and secondary safety and effectiveness endpoints, PFA with FARAPULSE also demonstrated an advantage in procedural efficiency. All operators (except at one centre) had no prior clinical experience with the pulsed field ablation system, but were considered expert operators with the thermal technologies. FARAPULSE AF ablation procedure times were significantly shorter with less variability than thermal procedures.
PV durability in re-ablation
Repeat ablation was performed in 4.6% of FARAPULSE patients and 6.6% in thermal ablation patients. The PV durability in re-ablated patients was 64.8% per vein (28.6% per patient) for FARAPULSE and 64.9% per vein (26.3% per patient) for thermal.
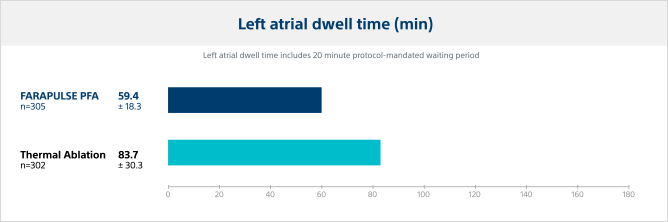
Left atrial (LA) dwell time
Pulsed field ablation catheter LA dwell time was significantly lower, reducing the LA dwell time by 29% versus thermal ablation modalities.
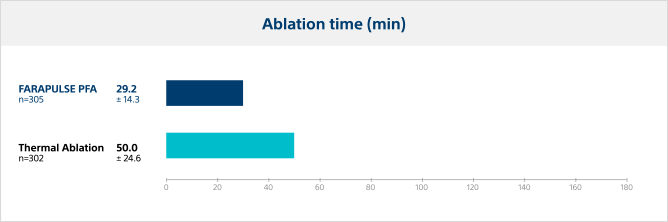
Ablation time
The time from first ablation to last ablation was significantly lower for PFA, with an average reduction in ablation time of 42% compared to thermal ablation modalities.
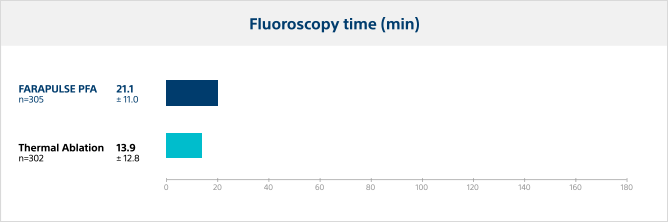
Fluoroscopy time
Pulsed field ablation required a longer duration of fluoroscopy versus thermal ablation, as expected with operators who are new to the PFA system.
In the ADVENT Pivotal Trial, FARAPULSE PFA procedure times were significantly shorter with less variability than thermal ablation.
*Bayesian credible interval (BCI) does not contain zero
Across multiple parameters of investigation, the ADVENT Pivotal Trial’s design is more clinically rigorous than any other pulsed field ablation study conducted to date.
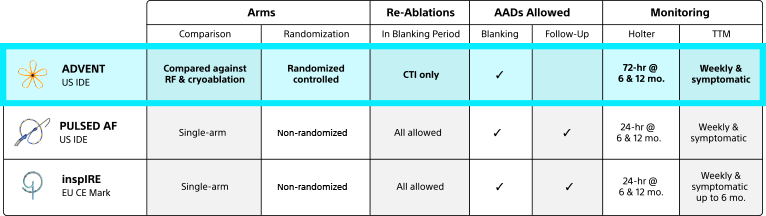
ADVENT study overview3

Blanking period (90 days)
- Re-ablations: only cavotricuspid isthmus ablation (CTI) ablations allowed, as needed
- AADs: allowed, as needed (Amiodarone was not allowed at any point)
Follow-up (at 12 months post-op)
- Re-ablations: none allowed
- AADs: use of Class I/III AADs not allowed after blanking
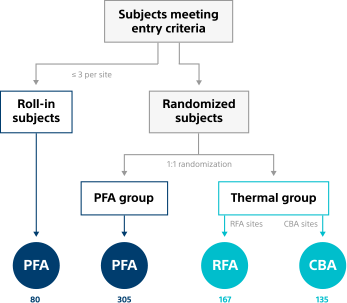
Study enrollment3
An initial sample size of 706 patients was used (626 randomised; 80 roll-in), leading to a patient modified Intent-to-Treat (mITT) cohort of 607 for primary results. mITT subjects received energy delivery for PVI with the randomised endocardial ablation catheter at an Index/Rescheduled Index Procedure.
Patients receiving treatment were divided across non-thermal (n=305) and thermal (302 total; RFA n=167, CBA n=135) arms.
Safety
Primary safety endpoint was a composite of pre-defined SAEs that were related to the use of the ablation catheter or ablation procedure occurring within 7 days of the index procedure. PV stenosis and atrio-esophageal fistula events were monitored to 12 months.
A secondary safety endpoint measured pulmonary vein (PV) narrowing and tested for superiority of PFA to thermal ablation. Changes in PV dimensions were measured at baseline and 90 days to characterize differences in PV remodeling and stenosis between PFA and thermal ablation.
Additional outcomes
- Silent cardiac event (SCE) and silent cerebral lesion (SCL) rates
- Procedure durations
- Quality of life
- Learning curve effects
- Rate of re-ablation and PVI durability at re-ablation
- Early recurrence of AF
- Post-blanking cardioversions
- Arrhythmia hospitalizations


